 Here we go with the first chapter in the Product & Process Control chapter of the CQE Body of knowledge.
Here we go with the first chapter in the Product & Process Control chapter of the CQE Body of knowledge.
This chapter cover 2 Major Tools for Product & Process Control:
- The Control Plan
- Work Instructions
While this is the first chapter in a new pillar of the CQE Body of Knowledge, you might not be shocked to hear that this topic is highly related to the Product & Process Design pillar.
The tools discussed here should actually be created during the design phase, and then used, maintained and updated throughout the lifecycle of a product.
It’s also worth noting that this entire pillar on Product & Process Control is strongly linked to risk management.
We manage risk by controlling our products and processes.
Keep this in mind as you’re developing and implementing your product/process control strategies, that all control activities should be risk based.
Let’s get started with the Control Plan.
An Easy Starter Quiz
Hey before you invest of time reading this chapter, try the starter quiz.
[WpProQuiz 82]
If you do really well, then you head down to the final quiz at the bottom.
Control Plans
A Control Plan is a comprehensive living document which describes the actions required at each step in your process to control and monitor a process and product.
A control plan actually starts long before you go into production. It begins during Product/Process Development with the identification of Critical to Quality Attributes (CTQs) and is used during Design Validation & Process Validation.
Remember, your CTQ’s are Product Characteristics that have a strong relationship with the user safety, quality, reliability, or regulatory compliance.
Your CTQ’s should be identified during development, and assessed for their associated risk using a PFMEA or FTA.
In a nutshell, we control and monitor our processes to ensure that our CTQ’s are consistently met, and to mitigate the risk of a failure of a CTQ.
Because our control and monitoring strategy is risk based, then the appropriate control strategy should be based on the capability of our process for each CTQ.
Remember, process capability represents the “likelihood” within a PFMEA.
Process Capability of CTQ’s should be assessed during process design and confirmed during process validation.
Essentially, the Control Plan should be a comprehensive list of all CTQ’s, where you monitor and control each CTQ using a risk-based approach that accounts for the capability of the process, and the criticality of the attribute.
In addition to the CTQ’s associated with your product, your control plan can also include the critical process parameters associated with your process.
During process development, you should identify process parameters that have a strong influence/relationship with your CTQ’s. These critical process parameters should also be included with your control plan.
Below is an example of a control plan.
The format is somewhat irrelevant and varies wildly between industries and companies. What does matter is the content.
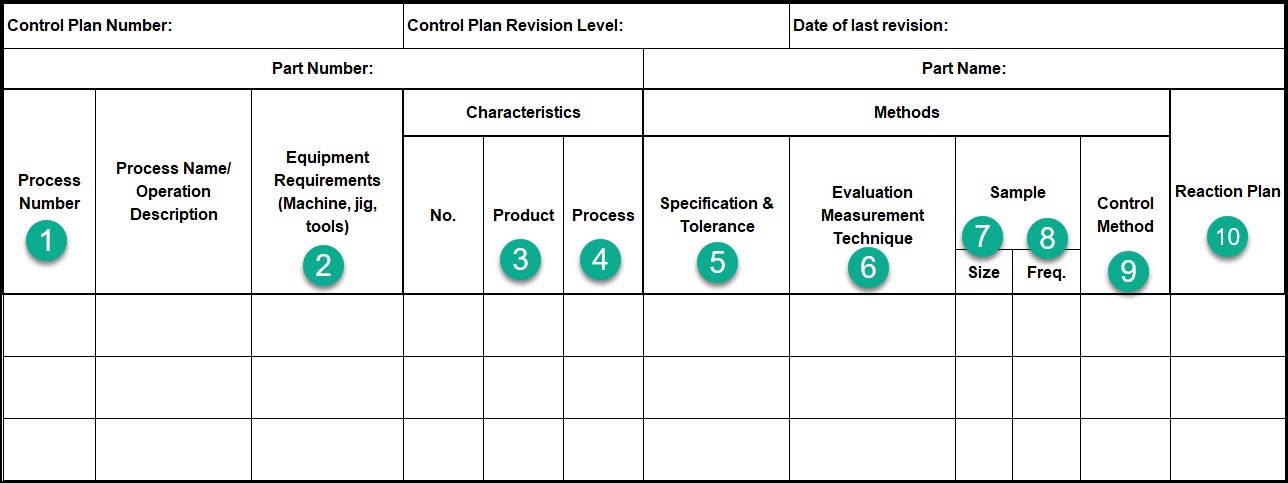
Let’s go through some of these columns in detail. The rest are self-explanatory.
- Process Number – A reference that should link to the process flow diagram to identify critical control points.
- Equipment Name/Number – A list of the required equipment associated with this process step or product.
- Product Characteristic – The CTQ’s associated with the product
- Process Characteristic – The critical process parameters associated with your process
- Specification & Tolerance –The specifications & tolerances for your Product & Process Characteristics
- Evaluation Measurement Technique – The required measurement technique for assessing conformance of a Product/Process Characteristic. This might also define the requirement measurement equipment and test method.
- Sample Size – Defines how many samples are required to be taken
- Sample Frequency –Defines how often a sample should be taken
- Control Method – A description of how this Product or Process Characteristic will be controlled. This might include the work instructions, SPC, Go/No-Go gauges, Poka-yoke features, sampling plans, visual inspections, automated inspections, 100% inspections, destructive testing, etc.
- Reaction Plan – A brief description of the appropriate reaction if the Product or Process Characteristic is ever found to be out of specification. The details of this specific column will be discussed in more detail below.
The Process Number and Name column should be linked to your process flow diagram. These steps in the process that are critical to the control of the product or process are often called critical control points.
Similarly, your controls in your control plan should be referenced in your PFMEA and should influence the “Detection” rating for each failure mode and its associated CTQ.
Remember, it should be the RPN number in the PFMEA that should dictate the level of control applied throughout the process after considering the capability of the process, and the severity of the failure.
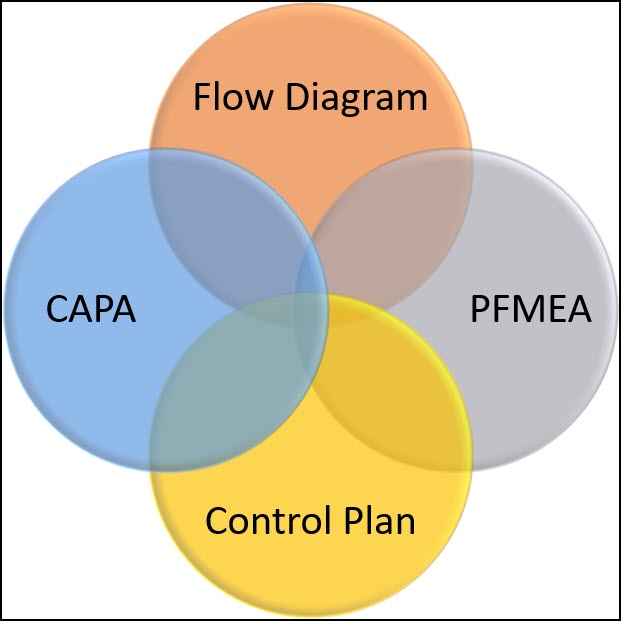
This is a risk-based approach to process control and monitoring.
Other links to the control plan includes the CAPA process which should act as a feedback mechanism to keep the control plan up to date.
As new failure modes are uncovered and new controls (Corrective Actions) put in place, this would drive a revision of the control plan and/or PFMEA.
Before we move on, I want to review the most important part of the Control Plan, the Reaction Strategy.
The Reaction Plan
Okay, so here’s how I typical reaction to a failure. It’s a real roller-coaster of emotions.
Failures in a process can often be stressful, frustrating, and chaotic situations.
The Reaction Plan is an over-looked aspect of the control plan, yet it is probably the most important because it helps you consistently perform in these stressful situations.
The goal of a reaction plan is to instruct the people executing the process how to properly respond to failures.
A good reaction plan is made up of 4 key steps, which should be intertwined with the CAPA process:
- Containment
- Diagnosis
- Verification
- Disposition
When a process is out of control, and found to be producing non-conforming product, the CAPA process should be initiated.
Reaction Step 1 – Containment
Step 1 in the reaction plan is to contain all potentially affected product. This activity should be conducted immediately after the issue is identified.
In this step you should identify, quarantine and segregate all potentially affected product (suspected product). A starting assumption of the suspected product should be all material built since the previous inspection.
These specific containment directions should be specified in the reaction strategy so that the containment actions can be immediately taken.
This step should also likely include an escalation to the appropriate folks, examples might include Quality, Engineering, Maintenance, etc.
Reaction Step 2 – Diagnosis
Step 2 is to diagnose the root cause of the issue. Depending on the complexity of the problem, this might be a cross-functional, in-depth investigation.
For simple and recurring issues this might include documented steps in the reaction plan to identify the root cause.
For complex failure modes, the CAPA process along with root cause analysis tools like the Fishbone Diagram and the 5-why analysis should be used.
Understanding the root cause should also come with an assessment of the risk. The disposition of affected product, and the depth of the corrective actions should all be risk based.
This step should also any actions required to get the process back into a state of control so that the process can continue.
Determining the root cause of the problem should also lead to the confirmation of the scope of affected material.
If the exact “start” of the issue cannot be identified, you might have to assume that all product since the last known inspection is affected.
Further testing or inspection of quarantined material can be conducted to identify when the issue likely started. Further inspection can also assist in root cause analysis.
Reaction Step 3 – Verification
So, you’ve assessed the root cause, and made corrections to the process to get it back into a state of control.
Before you start up again, you should verify that the problem has truly been fixed.
Step 3 in the reaction process is a verification step to ensure that the process is back in control. This usually means a re-measurement of the original feature that failed.
This can also include taking other measurements to ensure the correction didn’t have any negative unintended consequences for other CTQ’s.
Reaction Step 4 – Disposition
Once you know the root cause, and you’ve assessed the risk of the failure, it’s time to disposition the effected product.
Possible dispositions include scrap, sort, rework, use-as-is (UAI) or return-to-vendor (RTV).
Work Instructions

My whole life I’ve been building Lego’s and it wouldn’t have been possible without those fantastic work instructions they provide.
Work Instructions are easily the most commonly used Control tool, and there’s a reason why – they are can be very effective.
We first discussed work instructions in the Documentation of the Quality System and you’ll see it again when we talk about Lean Tools (Standard Work).
Work Instructions are a living document that represents the best, safest, most effective and efficient method for conducting an activity or executing a process.
Work Instructions defines the Current State of a process and should be created and owned by the people who do the work.
Work Instructions should describe the Who, What, When, Where, How and Why associated with the process. This document should contain the necessary detail and sequencing of activities to ensure consistent execution over time.
Work Instructions should always be displayed at the workplace and available to the folks doing the work.
Your Work Instructions may cover many of the following details:
- The equipment and tools required
- Equipment setup instructions
- The environment or location associated with the work
- Safety alerts for the employees
- A cross-reference to any other required processes or work instructions
- The critical process parameters associated with the process
- The critical to quality characteristics (CTQs) associated with the process
- Equipment maintenance procedures
- Test Methods for CTQWs
- Other non-product related criteria for the final product
- Workmanship Standards
- Material Handling Standards
- Images of expected conditions
- Images of common failure modes
- reaction strategies for common failure modes
Similar to a control plan, your work instructions should be a living document that are constantly being updated as new process information is learned.
Conclusion
Alright, that was short and sweet.
Okay, so we covered the 2 Major Tools for Product & Process Control:
- The Control Plan
- Work Instructions
A Control Plan is a comprehensive living document which describes the actions required at each step in your process to control and monitor a process and product.
A control plan starts during Product/Process Development with the identification of Critical to Quality Attributes (CTQs).
The Control Plan should be a comprehensive list of all CTQ’s, where you monitor and control each CTQ using a risk-based approach that accounts for the capability of the process, and the criticality of the attribute.
One of the most important aspects of a control plan is the Reaction Plan.
The goal of a reaction plan is to instruct the people executing the process how to properly respond to failures.
A good reaction plan is made up of 4 key steps, which should also be used with the CAPA process:
- Containment – Step 1 in the reaction plan is to immediately identify and quarantine all potentially affected product.
- Diagnosis – Step 2 is to investigate and diagnose the root cause of the failure, and initiate corrective actions.
- Verification – Step 3 is to verify your actions were effective in moving the process back into a controlled state.
- Disposition – The final step in the reaction process is to disposition the affected material (Scrap, Sort, Rework, etc)
Work Instructions are the second and final product/process control tool.
Work Instructions are a living document that represents the best, safest, most effective and efficient method for conducting an activity or executing a process.
Work Instructions defines the Current State of a process and should be created and owned by the people who do the work.
Work Instructions should describe the Who, What, When, Where, How and Why associated with the process. This document should contain the necessary detail and sequencing of activities to ensure consistent execution over time.
The Final Quiz
[WpProQuiz 83]