 The Quality System is the second pillar of the CQE Body of Knowledge and it contains 6 main chapters within it:
The Quality System is the second pillar of the CQE Body of Knowledge and it contains 6 main chapters within it:
- Elements of the Quality System
- Documentation of the Quality System
- Quality Standards & Other Guidelines
- Quality Audits
- Cost of Quality
- Quality Training
Similar to the Management & Leadership portion of the exam, it is important to remember that while the Quality System is 1/6th of the total material within the body of knowledge, it actually only comprises 15 questions out of the total 160 or 10% of the exam.
For each of the chapters above, you’ll find a quick summary below along with a link to the full chapter.
Elements of the Quality System
Quality is your ability to continually satisfy and exceed your customers needs and expectation.
All Quality activities are meant to assures that you’re providing value to your customers.
To effectively achieve high levels of Quality, your Quality System must shadow all phases of your products life cycle – all the way from the early research and design phases to the final distribution and eventual decommissioning of the product.
Below is the Learning Objective for the Elements of the Quality System portion of the ASQ CQE Body of Knowledge:
- Define, describe, and interpret the basic elements of a quality system, including planning, control, and improvement, from product and process design through quality cost systems, audit programs, etc. (Evaluate)
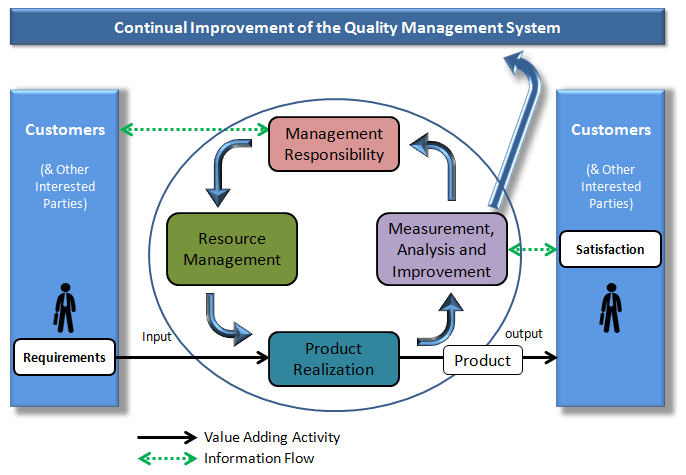 Your Quality System is the collection of the interrelated processes and activities that are meant to ensure that your product or service meets the needs & requirements of the customer.
Your Quality System is the collection of the interrelated processes and activities that are meant to ensure that your product or service meets the needs & requirements of the customer.
This chapter discusses the ISO framework for the Quality System which include the basic elements of a Quality System.
Documentation of the Quality System
You may not believe this but. . . Documentation is a strategic business advantage.
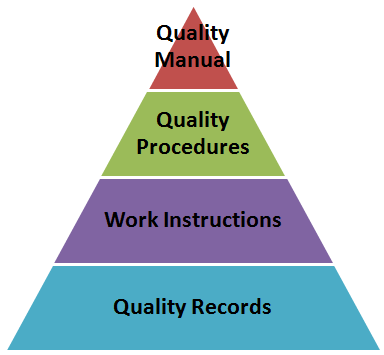
It is extremely easy to overlook the fact that Documentation plays a key role in the Quality Management System.
Documentation is a tool that captures your intentions for Quality and then cascades those intentions down into every level of the organization.
Documentation also allows you to capture your organizations best practices & tribal knowledge into a “memory bank.” This prevents all the lessons learned over time from leaving when the employees walk out the door.
Effective execution of your Documentation System lead to a higher level of control over your critical process. Effective documentation also results in employee who are performing their job safely and with an adequate amount of information which leads to a sustained level of high quality.
This is exactly why documentation should NOT be viewed as simply a regulatory requirement that lacks any real value.
 Below is the Learning Objective for The Documentation of the Quality System portion of the ASQ CQE Body of Knowledge:
Below is the Learning Objective for The Documentation of the Quality System portion of the ASQ CQE Body of Knowledge:
- Identify and apply quality system documentation components, including quality policies, procedures to support the system, configuration management and document control to manage work instructions, quality records, etc. (Apply)
Quality Standards & Other Guidelines
Below is the Learning Objective for The Quality Standards & Other Guidelines portion of the ASQ CQE Body of Knowledge:
- Describe key points of the ISO 9000 series of standards and how they are used. [Note: Industry-specific standards will not be tested.] (Apply)
- Define and distinguish between national and international standards and other requirements and guidelines, including the Malcolm Baldrige National Quality Award (MBNQA)
ISO 9000:2015 – Quality Management Systems – Vocabulary & Fundamentals
ISO 9000 is the first of the three documents in the series and it sets the foundation for the entire series by defining the fundamentals of Quality Management Systems.
ISO 9001: 2015 – Quality Management Systems – Requirements
ISO 9001 is that standard that defines the requirements that an organization must be compliant with in order to be “ISO Certified”. An organization does not necessarily need to be in conformance with ISO 9000 or 9004 to be ISO certified, only ISO 9001 must be fully met for certification.
ISO 9004- Managing for the Sustained Success of an Organization – a Quality Management Approach
Where ISO 9001 is compliance based and full of requirements, ISO 9004 is improvement based and is focused on efficiency.
The content of ISO 9004 are not requirements that your organization must meet, they’re guidelines that can be implemented to improve the performance & efficiency of your Quality Management System.
MBNQA (Malcolm Baldrige National Quality Award)
The Malcolm Baldrige National Quality Award seeks to recognize businesses who demonstrate exemplary performance in all areas of Quality.
There are 7 different criteria, below, that are used in determining which organization should win the award, and reflect the core values of the award. These criteria are based on a company’s results obtained in these areas:
- Leadership
- Workforce Focus
- Process Management
- Strategic Planning
- Measurement, Analysis, & Knowledge Management
- Customer Focus
- Business Results
Quality Audits
An audit can be described as formal, systematic, independent & fact based process of collecting objective evidence to assess the extent to which the auditee is in compliance with the audit criteria (requirement or standard).
There are 4 topic areas associated with Quality Auditing that you should understand.
- The Types of Audits
- The Roles & Responsibilities in Auditing
- Audit Planning & Implementation
- Audit Reporting & Follow up
Type of Audits
There are 3 different ways to classify an audit; by the relationship of the parties involved, by the focus of the audit or by the purpose of the Audit.
We also learned that these types of audits are not mutually exclusive and simply represent similar audits from different perspectives.
Roles & Responsibilities
There are 3 primary roles that are normally involved in Quality Audits; the Auditors (and Lead Auditor), the Auditee & the Client. As a Quality Engineer you will find yourself playing any of those roles and you must know what the Responsibilities are for each.
Audit Planning & Implementation
To ensure that your Quality Audits are effective and efficient, you must understand the best practices around Audit Planning & Execution. This includes the importance of an audit plan, along with the details of a typical audit plan.
Next, this section detailed the typical audit process, which normally follow the linear process shown below:
- The Opening Meeting
- The Data Collection Phase of the Audit
- The Closing Meeting
Audit Reporting & Follow Up
Finally, this chapter discussed the fourth key area of a Quality Audit which include the Audit Reporting and Follow Up.
This section details the process of creating an Audit Report, and the appropriate level of follow up required from the Auditor, Auditee & Client to ensure that effective corrective actions are implemented to address any audit observations.
Cost of Quality
Philip Crosby once said “Money is the language of management; you need to show them the numbers.” Since then, the Cost of Quality concept has been continuously improved into a fully developed financial model that has many strategic benefits.
This Chapter will break down The Cost of Quality into its key concepts, which include:
- The Total Cost of Quality & the 4 Quality Cost Categories
- Collection & Reporting of Quality Cost Data
- The COQ Benefits & Limitations
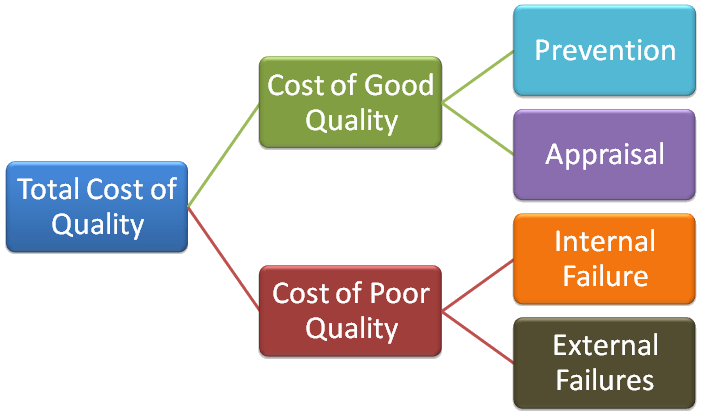
4 Quality Cost Categories – Total Quality Cost
When it comes to Quality & Cost, there are 4 different Categories that can be utilized to capture your quality related costs, these are which are Prevention Cost, Appraisal Cost, Internal Failure Cost, External Failure Cost.
The Total Quality Cost then is simply the sum of all these cost categories.
Quality Cost Data Collection & Reporting
Adopting a Cost of Quality program is an excellent way to align your business results of profitability to your Quality efforts. This COQ Program is achieved through 3 general steps.
- Step 1 – Define your COQ Categories & Data Collection Method
- Step 2 – Define your Data Collection Method & Responsible Person
- Step 3 – Collect, analyze & report Quality Cost data and drive improvements.
COQ Benefits & Limitations
We also discussed the benefits of a COQ program, which include:
- providing cost-benefit justification for needed Corrective Actions & Improvement projects.
- quantifying the costs associated with inefficient or incapable processes that result in unwanted variation & waste.
- highlighting the importance of Prevention activities as an investment in cost avoidance, and as a method to reducing quality costs.
Finally we discussed the limitations of a COQ program which include the fact that a COQ program by itself does not lead to improvement. Another limitation of the Quality Cost system is its inability to quantify the Hidden Quality Costs that every company experiences.
 Quality Training
Quality Training
Deming Said – “It’s not enough to do your best; you must know what to do and then do your best.” Without the proper training, your world class systems can be made irrelevant.
In addition to preventing issues – training can also be utilized as a continuous improvement tool to improve performance. This is why ISO has specific requirements for employee training – because it is the linchpin between your process & your people.
Below is the Learning Objective for The Quality Training portion of the ASQ CQE Body of Knowledge:
- Identify and define key elements of a training program, including conducting a needs analysis, developing curricula and materials, and determining the program’s effectiveness. (Apply)
The training model presented here is called the ADDIE Model. It is not unique to Quality and can be applied to any other topic or situation.
Within these sections we also discussed the important of the Forgetting Curve, Spaced Repetition and Active Learning; and their impact on the retention of a new concept into long term memory.
YouTube Videos
I’ve put together a collection of videos that cover all of the topics within the Quality System into one Playlist, for your viewing pleasure!
Ready to see the next Major Pillars: Management & Leadership, Product & Process Design, Product & Process Control.
Have a comment question or concern? Please don’t hesitate to Contact Me.
